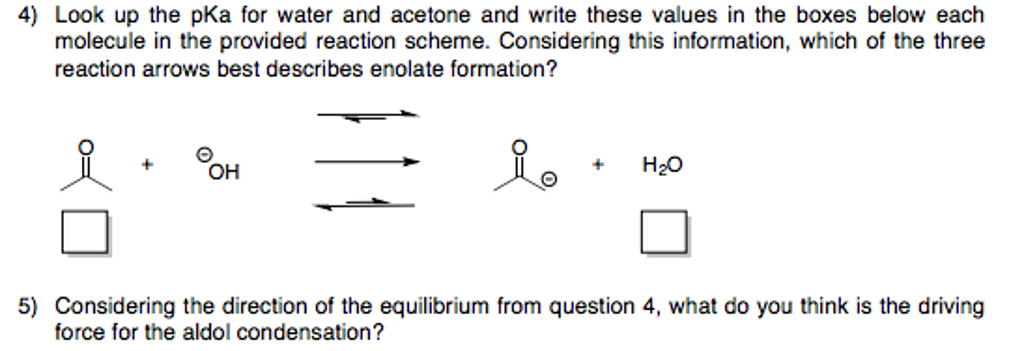
SOLVED: Calculate Ka values from the following pKa's: (a) Acetone, pKa = 19.3 (b) Formic acid, pKa = 3.75
If acetone has a pKa of 19, what ratio of enolate to acetone molecules would you expect at equilibrium? - Quora
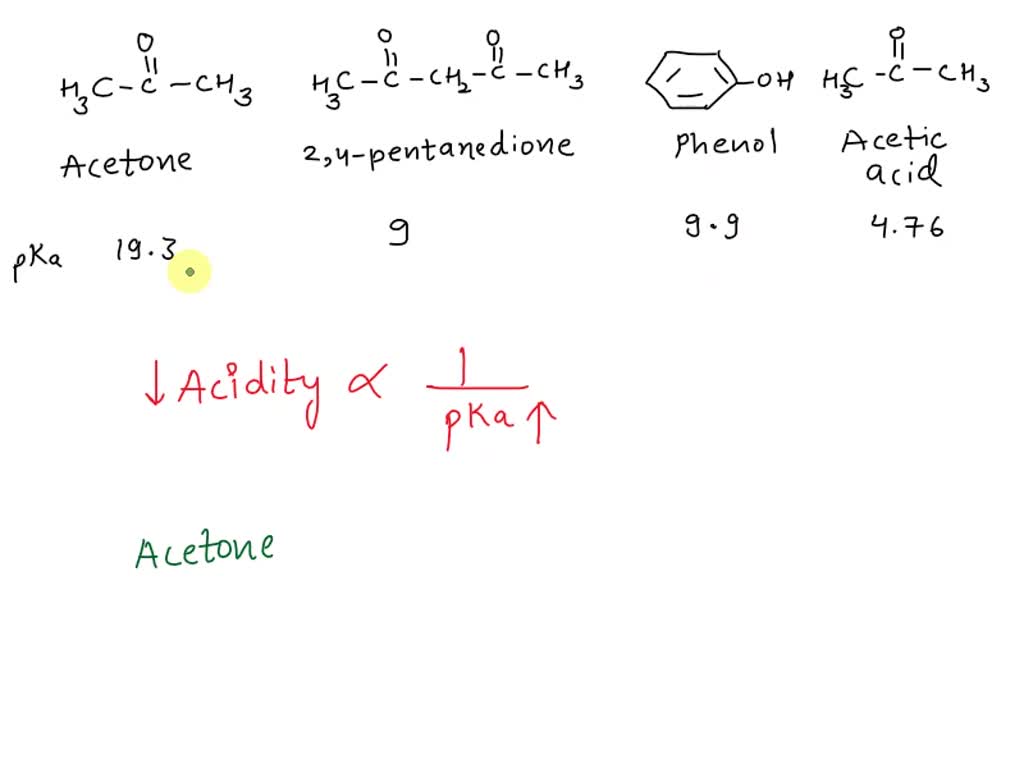
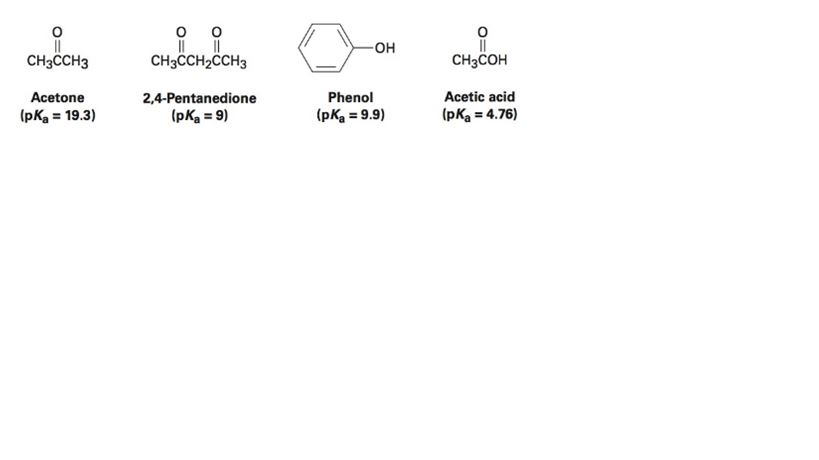
SOLVED: 2-44 Rank the following substances in order of increasing acidity: CH3CCH3 Acetone (pKa 19.3) CH3CCH2CCH3 2,4-Pentanedione (pKa = 9) OH CH3COH Phenol Acetic acid

Experimental pKa values and structures of the conformers of acetic,... | Download Scientific Diagram
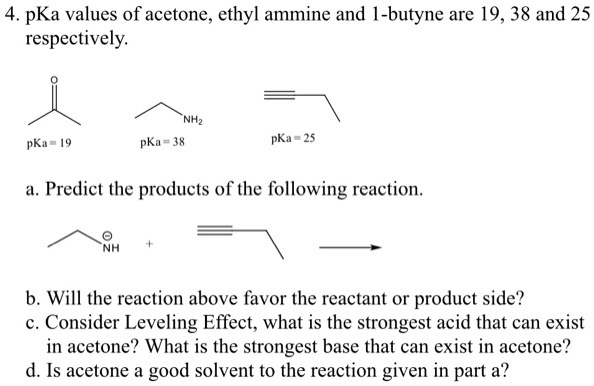
SOLVED: pKa values of acetone, ethyl ammine and -butyne are 19,38 and 25 respectively: NH; pKa = pKa = 38 pKa Predict the products of the following reaction b. Will the reaction
![College: Peptides and pKa] Are these the correct summary of charges for the peptide and pKa? : r/chemistryhomework College: Peptides and pKa] Are these the correct summary of charges for the peptide and pKa? : r/chemistryhomework](https://i.redd.it/wz5fjhe9c9n81.jpg)
College: Peptides and pKa] Are these the correct summary of charges for the peptide and pKa? : r/chemistryhomework

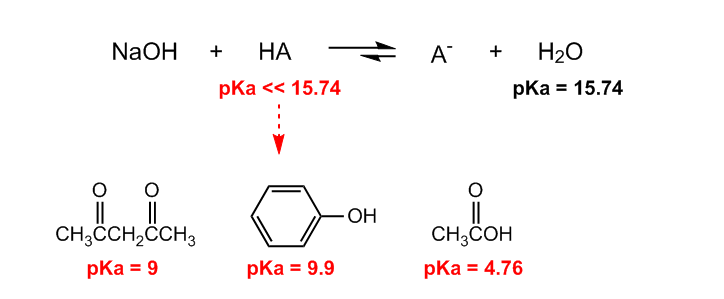
OneClass: Answer questions a-c about the Bronsted acid-base reaction below using the identifying lett...

A Theoretical Study of the Enol Contents of Cyclohexanone, Cyclopentanone and Acetone | Semantic Scholar
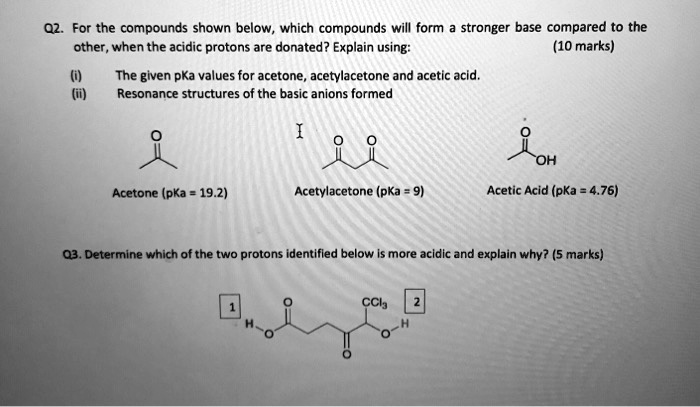
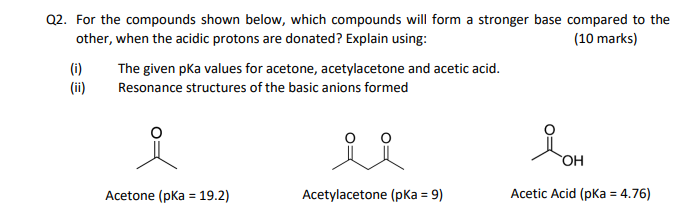
SOLVED: For the compounds shown below, which compounds will form a stronger base compared to the other when the acidic protons are donated? Explain using: (10 marks) The given pKa values for

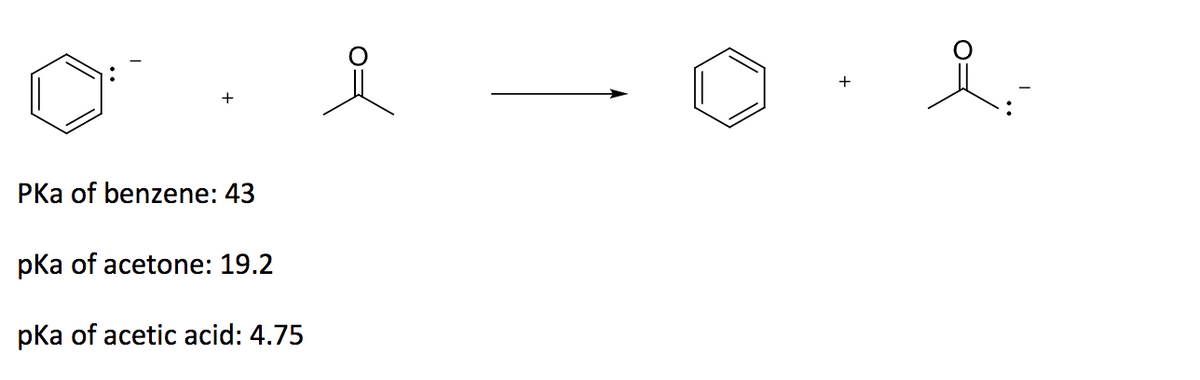

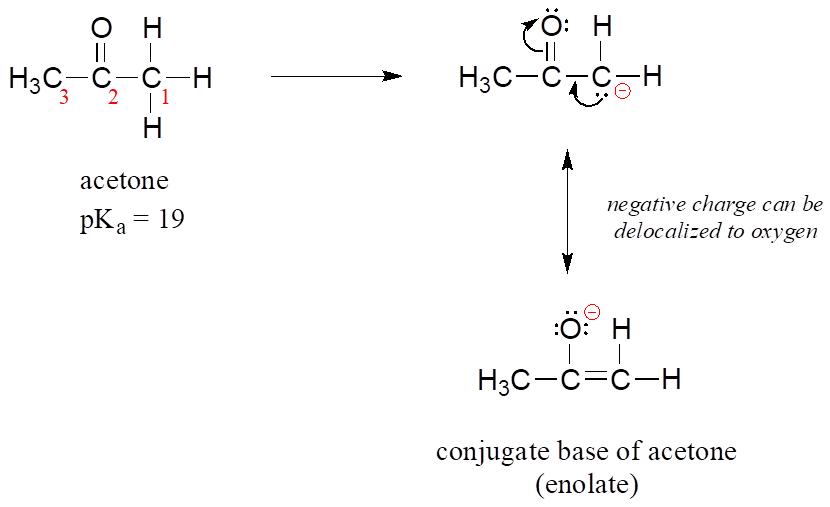

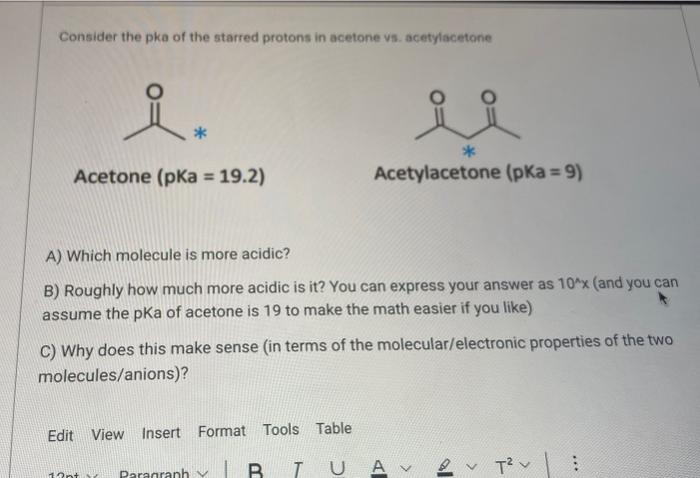
![Solved] As noted in Table 3.1, the pKa of acetone | SolutionInn Solved] As noted in Table 3.1, the pKa of acetone | SolutionInn](https://s3.amazonaws.com/si.question.images/image/images11/877-C-O-S(329).png)