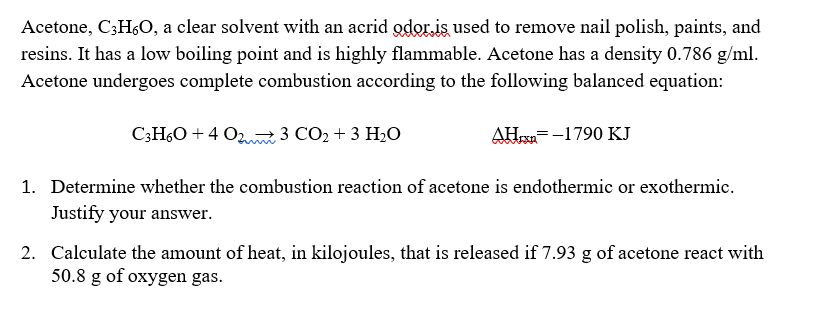
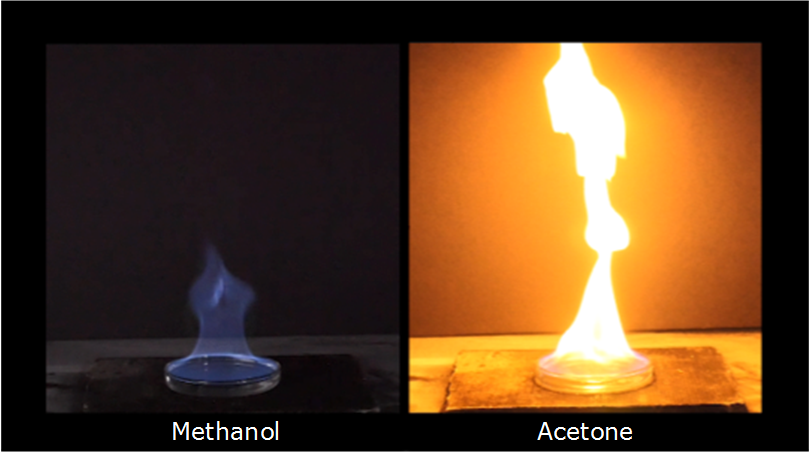
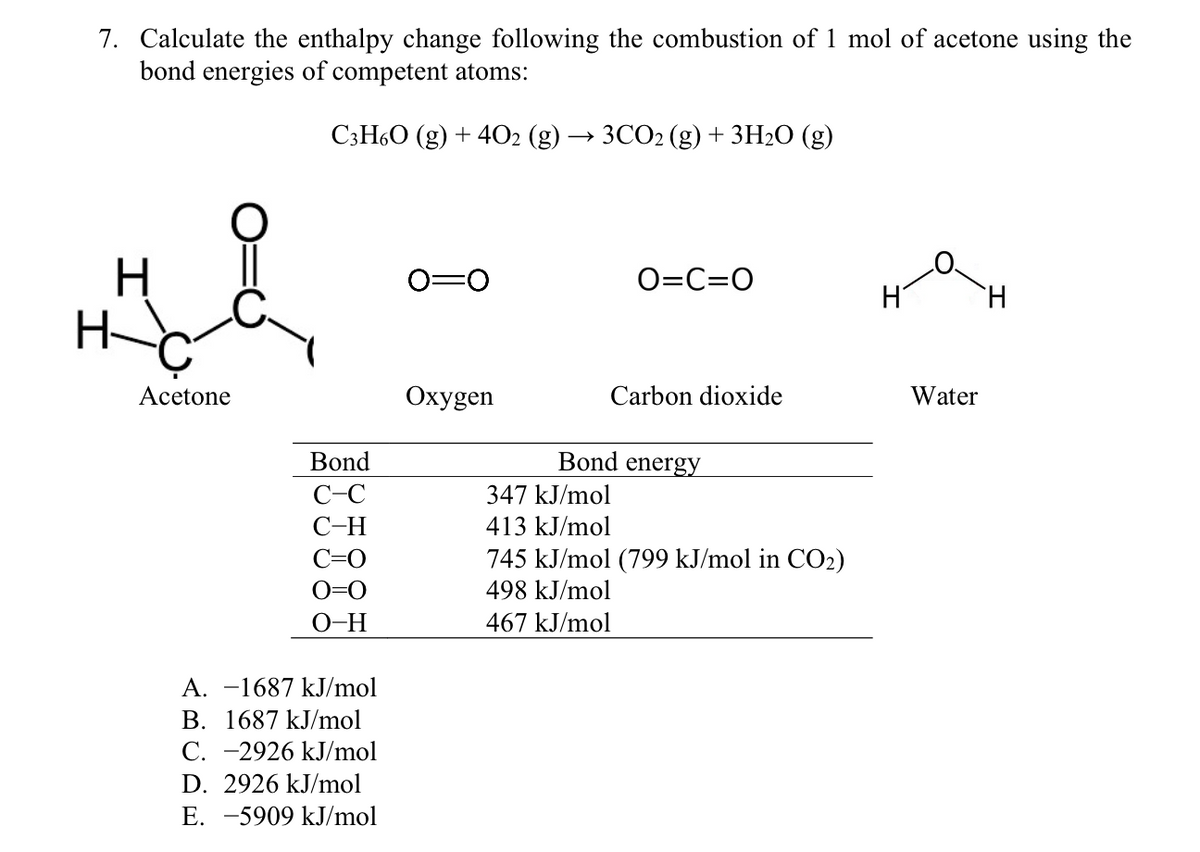
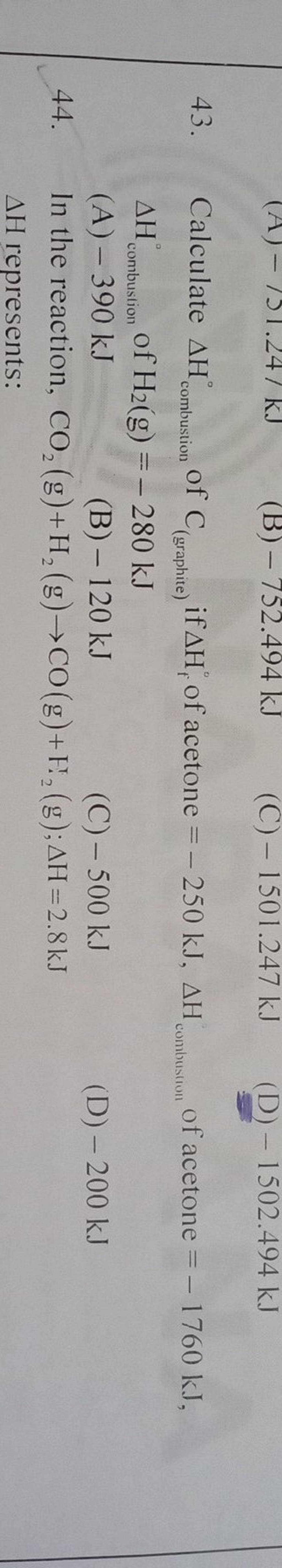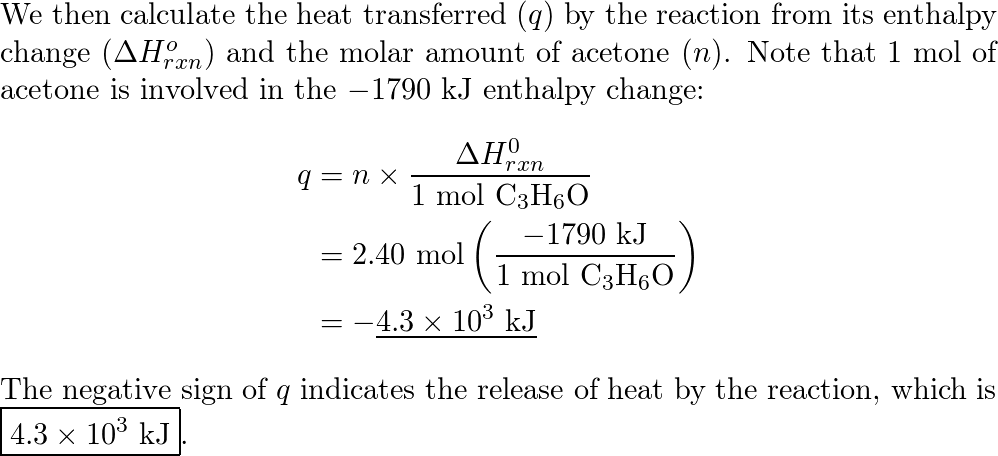Using the given data and calculate the enthalpy of formation of acetone(g). Bond enthalpy of : C - H = 415 ; C - C = 350 ; (C = O) =

SOLVED: Consider the following reaction involving the combustion of acetone (C3H6O): C3H6O (L) + 4 O2 (g) â†' 3 CO2 (g) + 3 H2O (l) ΔH = 1790 kJ. How much heat (

Calculate the volume of carbondioxide produced by the combustion of 40 ml of acetone vapours in presence of excess of oxygen?

OneClass: Acetone, CH3COCH3, is a liquid solvent. The enthalpy change at 25C and 1 atm for the comple...
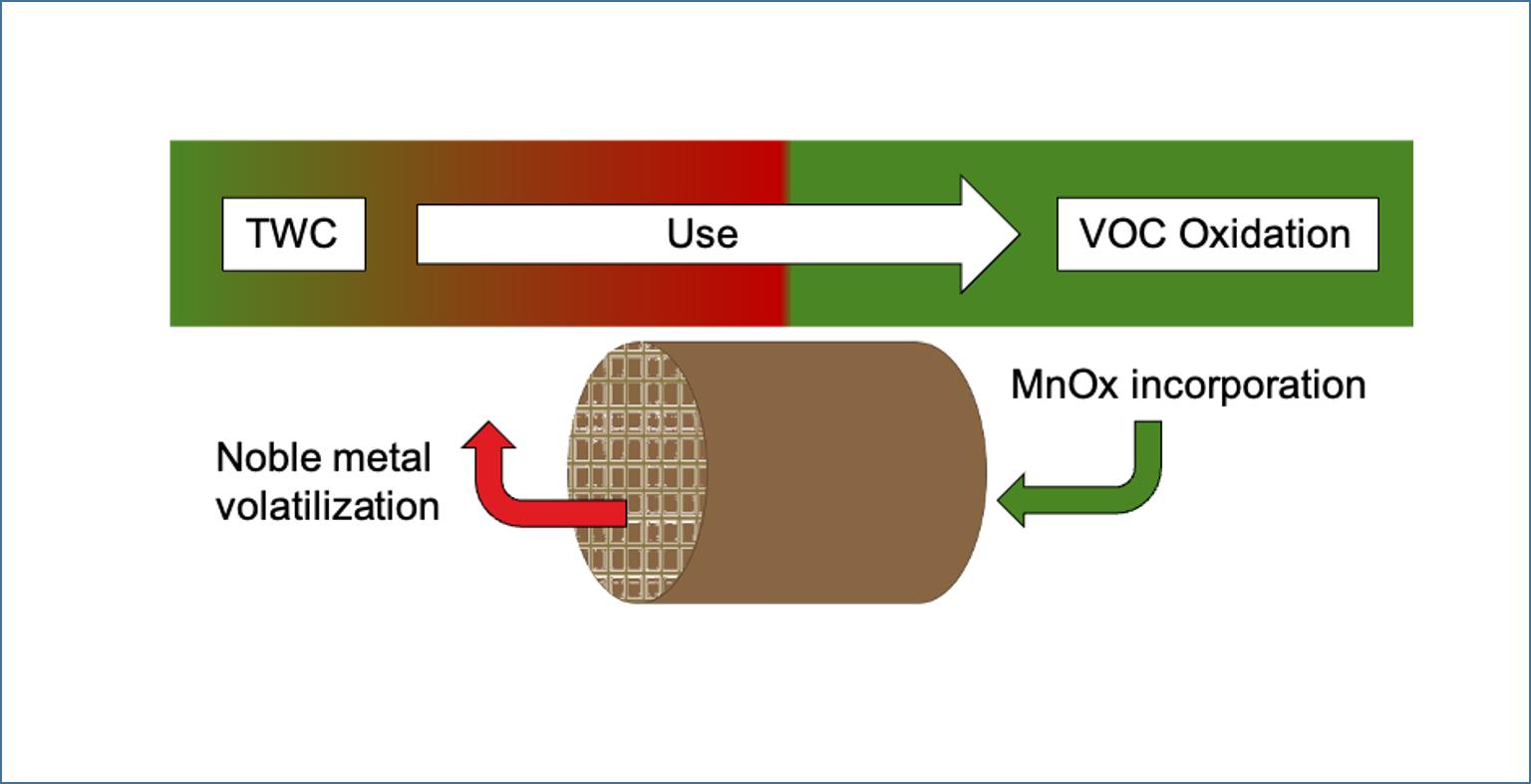
Catalysts | Free Full-Text | Valorization of Recycled Honeycombs from Exhausted TWCs by Means of Their Use as a Support of MnOx Catalysts for Acetone Combustion
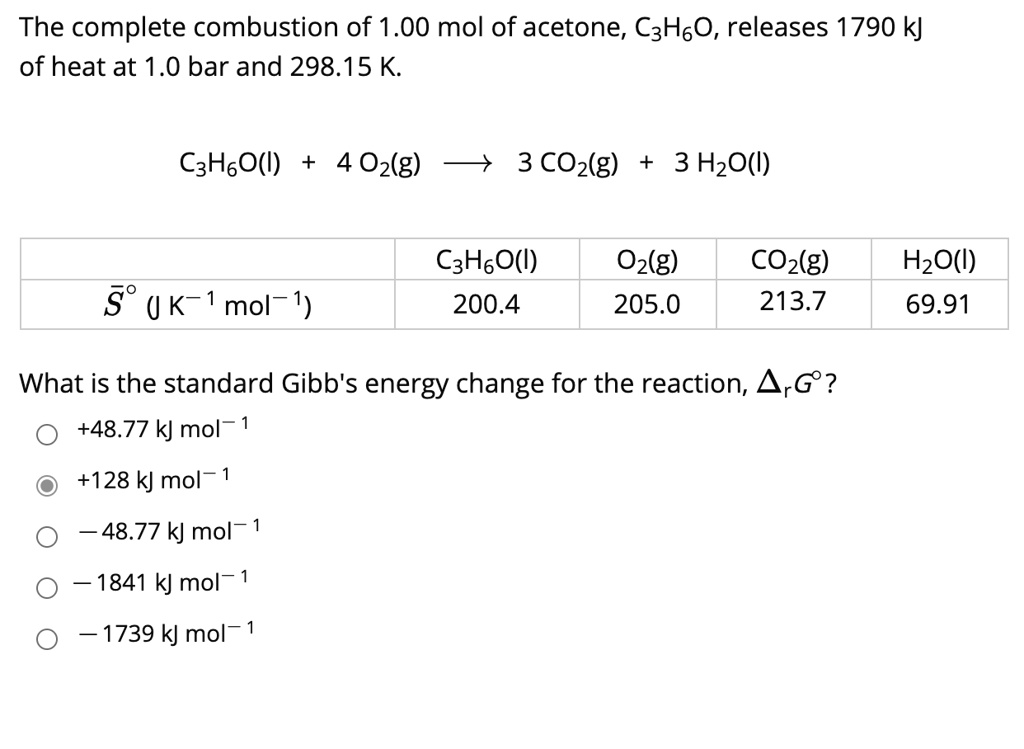
SOLVED: The complete combustion of 1.00 mol of acetone, C3H6O, releases 1790 kJ of heat at 1.0 bar and 298.15 K. C3H6O(g) + 4 O2(g) -> 3 CO2(g) + 3 H2O(g) C3H6O(g)
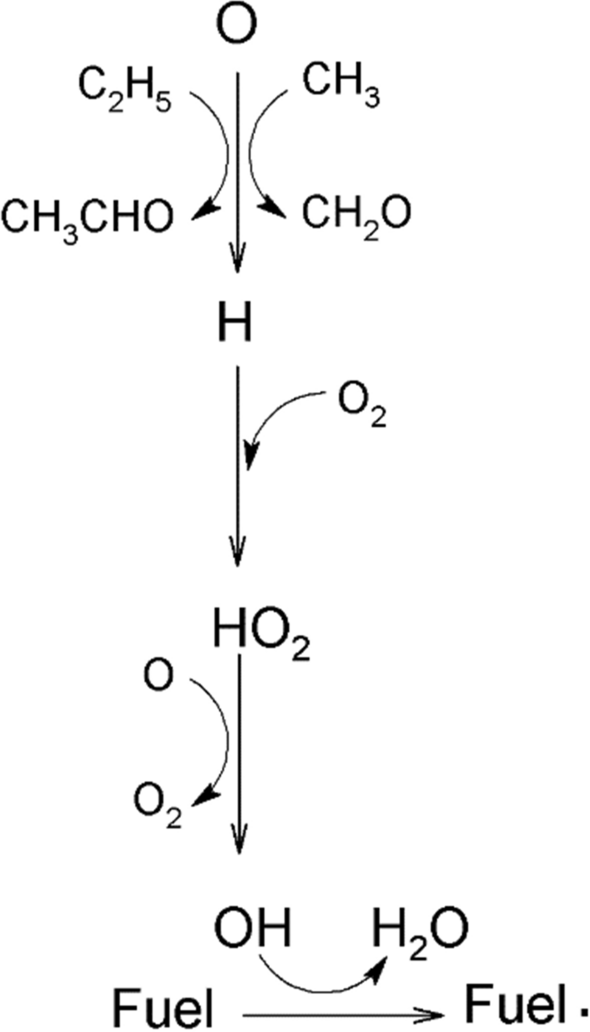
Kinetic analysis of the acetone-butanol-ethanol combustion mechanism in 0D simulated Otto cycle internal engine | SpringerLink

SOLVED: Complete combustion of acetone is given by: C3H6O(l) + 4O2 –> 3CO2(g) + 3H2O(l). ΔH for this reaction is -1790 kJ. ΔHfo for O2 is 0 kJ, for CO2(g) is -393.5
✓ Solved: Acetone, CH3COCH3, is a liquid solvent. The enthalpy change at 25^°C and 1 atm for the complete...

Calculate the volume of CO2 produced by the combustion of 40 mL of acetone in the presence of excess of oxygen.


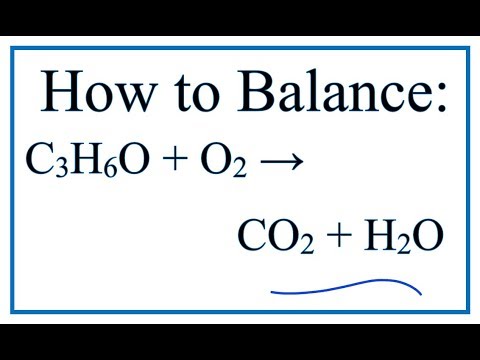


![ANSWERED] 59. Consider the thermochemical equation... - Inorganic Chemistry - Kunduz ANSWERED] 59. Consider the thermochemical equation... - Inorganic Chemistry - Kunduz](https://media.kunduz.com/media/sug-question/raw/57857865-1659726469.634809.jpeg)