The density of \( \mathrm{CaF}_{2} \) (fluorite structure) is \( 3.18 \mathrm{~g} / \mathrm{cm}^... - YouTube
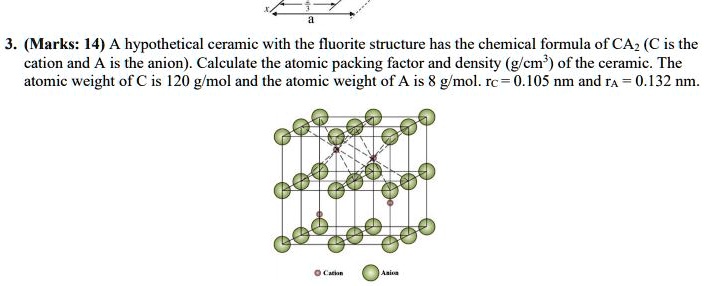
SOLVED: A hypothetical ceramic with the fluorite structure has the chemical formula of CAz (C is the cation and A is the anion). Calculate the atomic packing factor and density Cm of

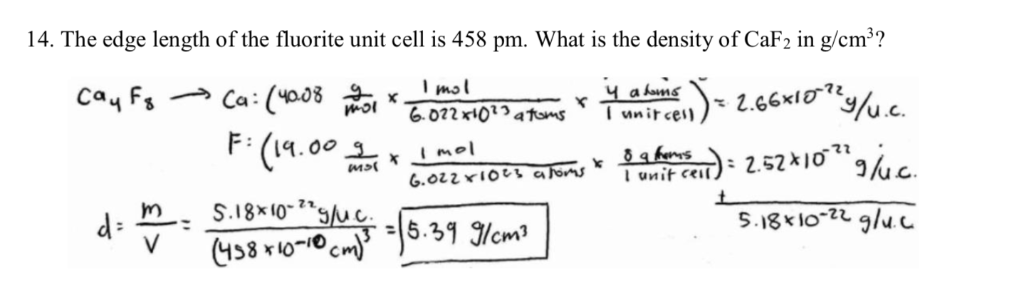
The density of CaF2 (fluorite structure) is 3.18 g/cm^3 . The length of the side of the unit cell is .

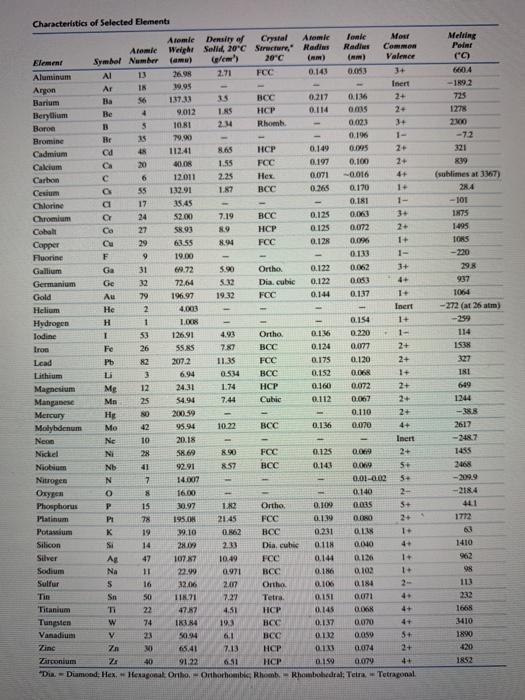
Problem 2: From the data in the table below, compute the theoretical density of C a F 2 which has the fluorite structure; | Homework.Study.com
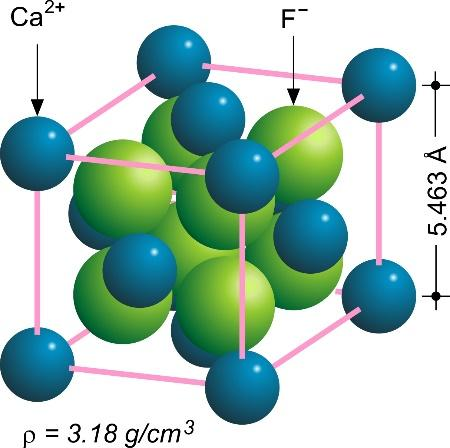
Minerals | Free Full-Text | A Density Functional Theory Study on the Effect of Lattice Impurities on the Electronic Structures and Reactivity of Fluorite

Minerals | Free Full-Text | The Influence of Surface Heterogeneity of Fluorite on the Adsorption of Alkyl Sulfonates
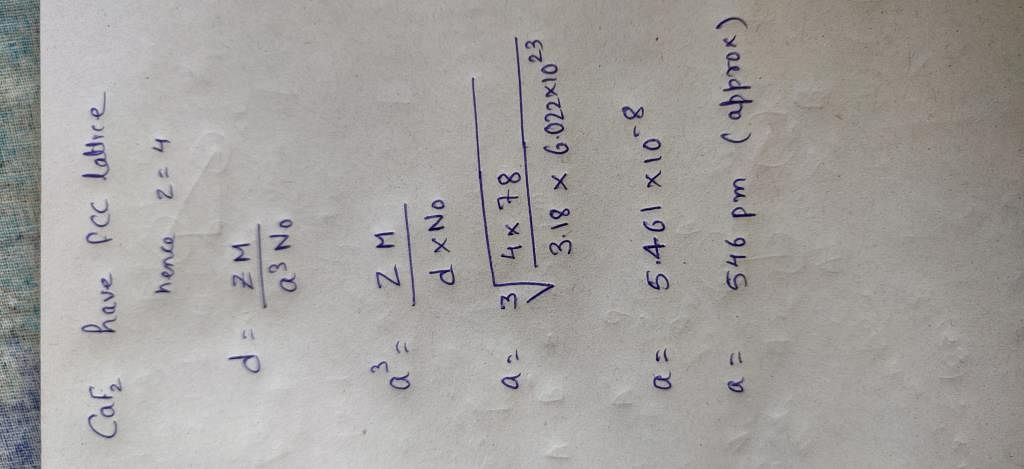
The density of CaF2 (fluorite structure) is 3.18 g/cm3. The length of the side of the unit cell is:a)253 pmb)344 pmc)546 pmd)273 pmCorrect answer is option 'C'. Can you explain this answer? -
The density of $ Ca{F_2} $ (fluorite structure) is 3.18 $ gc{m^{ - 3}} $ . The length of the side of the unit cell is:a)253 pmb)344 pmc)546 pmd)273 pm
The density of the CaF2 (fluorite structure) is 3.18 g/cm^3 . The length of the side of the unit cell is - Sarthaks eConnect | Largest Online Education Community

The Density Of Gemstones: Why Diamonds Rubies And Sapphires Are The Most Common | BeadWorksPhiladelphia.com

The density of `CaF_(2)` (flourtie structure ) is `3.18 g// cm^(3)`. The length of the side of - YouTube
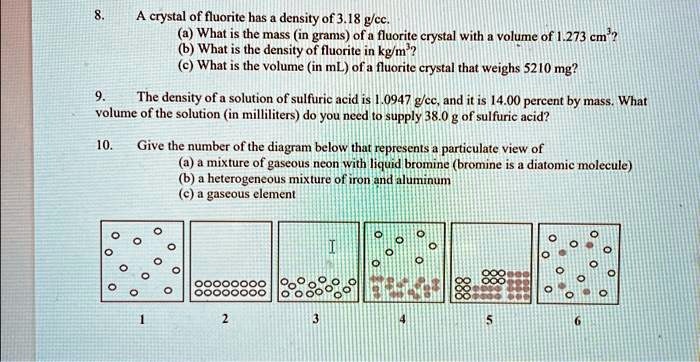
SOLVED: Texts: 8. A crystal of fluorite has a density of 3.18 g/cc. b) What is the density of fluorite in kg/m³? c) What is the volume (in mL) of a fluorite

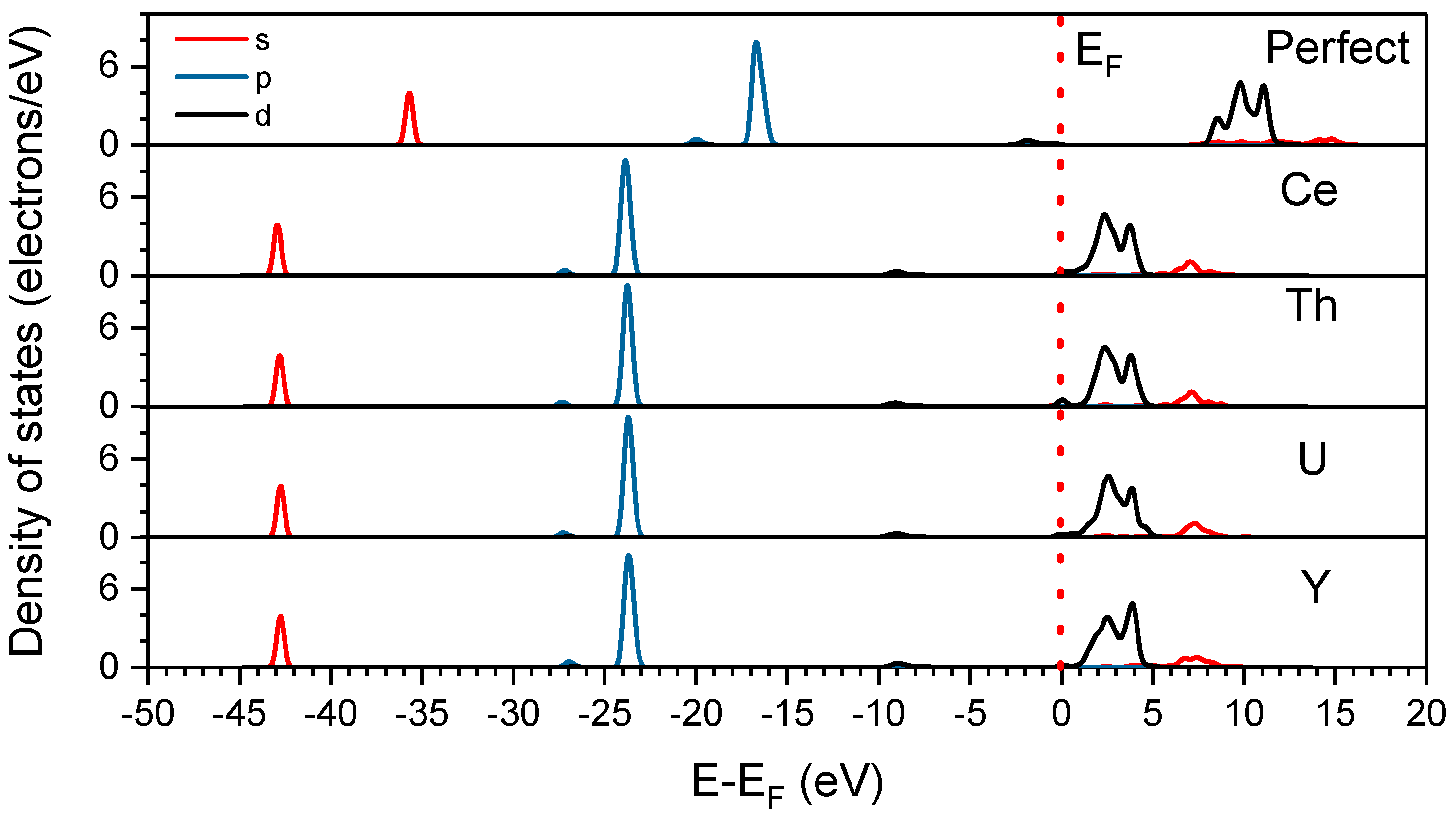
Electron density of states (DOS) of UO 2 with the fluorite structure... | Download Scientific Diagram