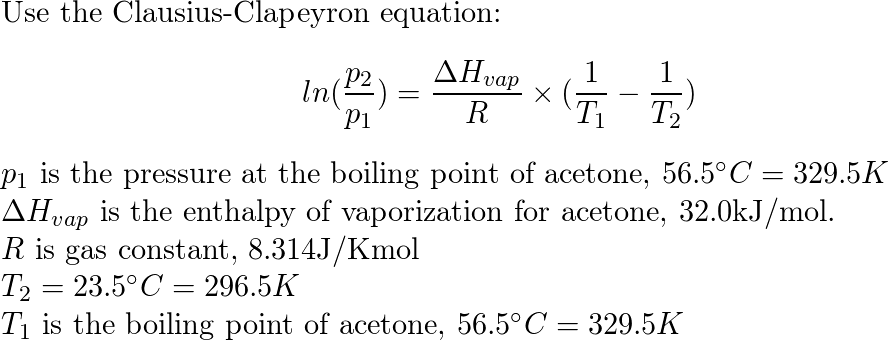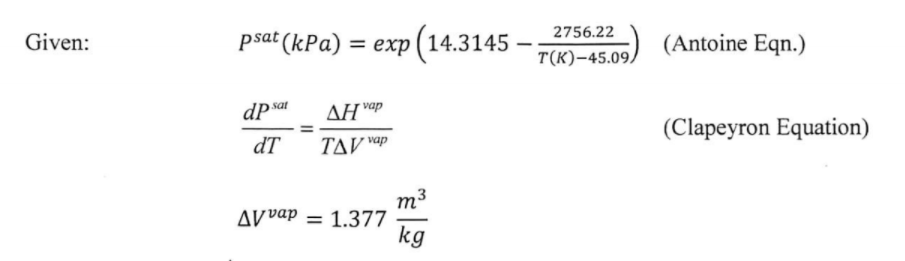How to calculate the vapor pressure of acetone at 25.0°C if the enthalpy of vaporization for acetone is 32.0 kJ/mol and the normal boiling point of acetone is 56.5°C - Quora

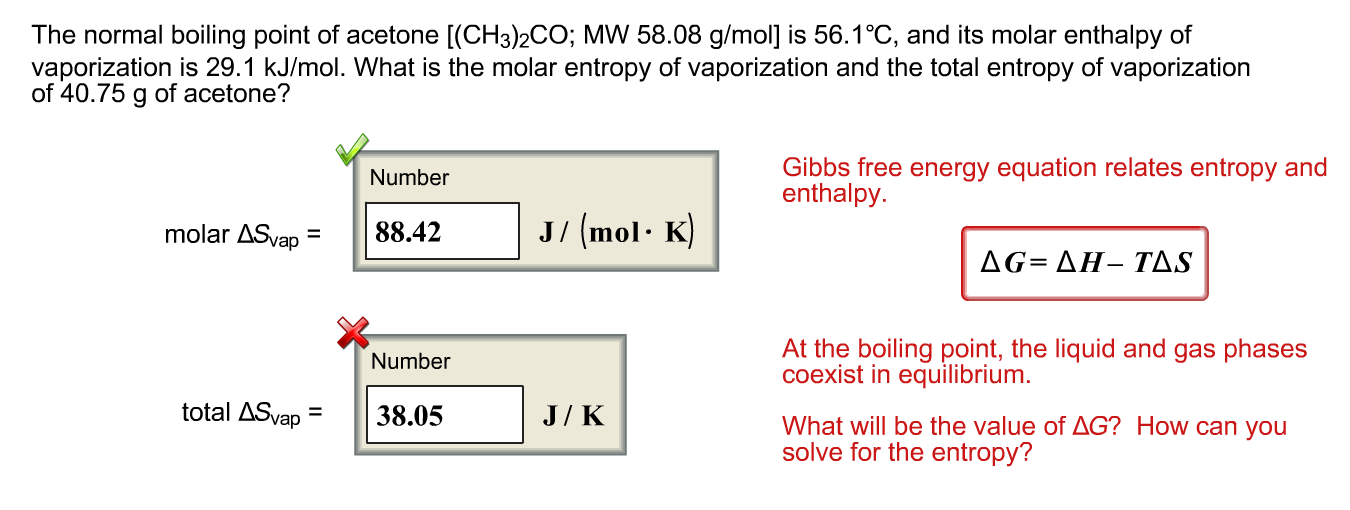
OneClass: A standard entropy of vaporization of acetone is approximately 85jk/mol at its boiling poin...
How to calculate the vapor pressure of acetone at 25.0°C if the enthalpy of vaporization for acetone is 32.0 kJ/mol and the normal boiling point of acetone is 56.5°C - Quora
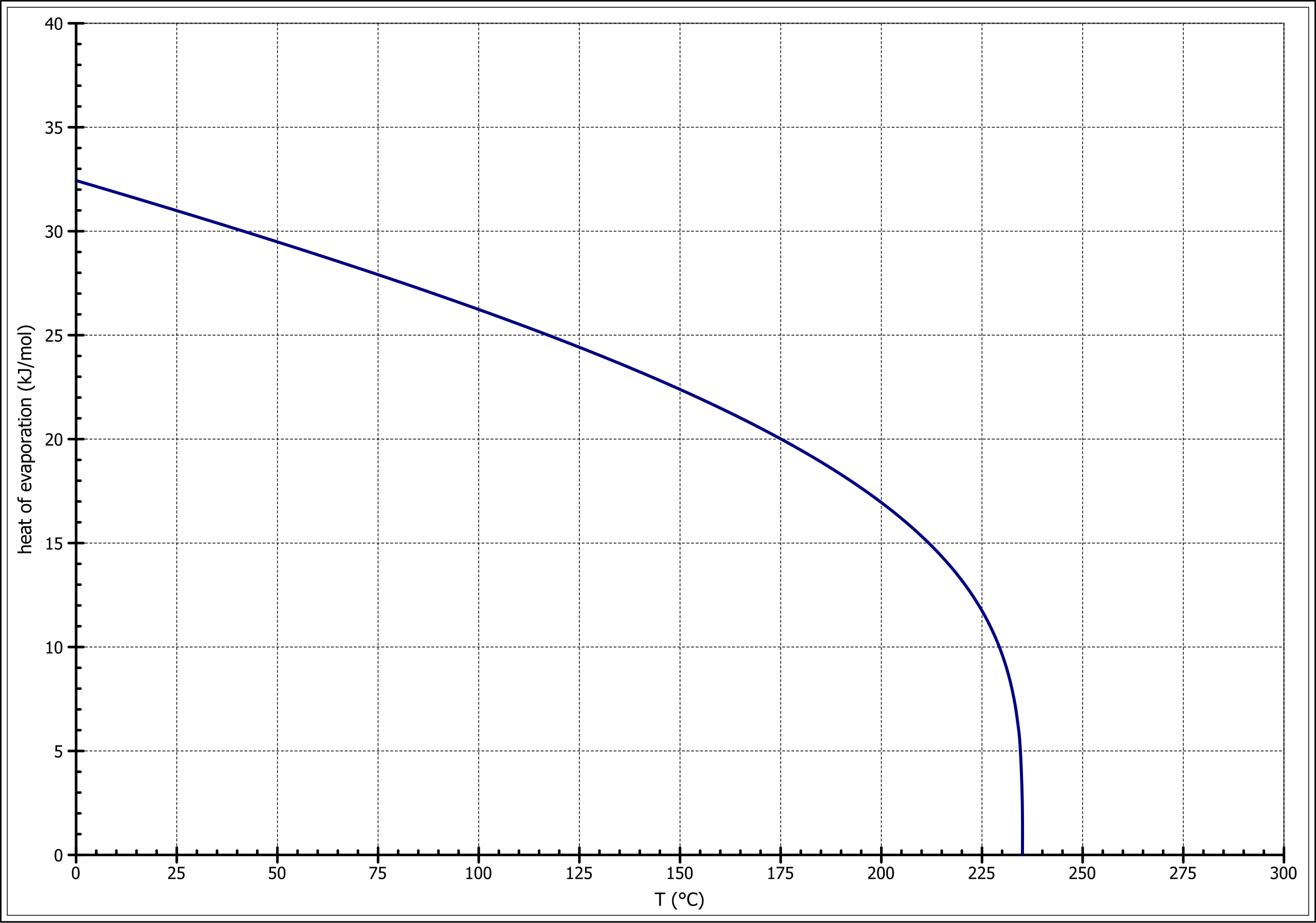
A Theoretical Analysis on Enthalpy of Vaporization: Temperature-Dependence and Singularity at the Critical State Abstract 1. Int

The enthalpy of vaporization of water at 100^o C is 40.63 KJ mol^-1 . The value Δ E for this process would be:
A Theoretical Analysis on Enthalpy of Vaporization: Temperature-Dependence and Singularity at the Critical State Abstract 1. Int

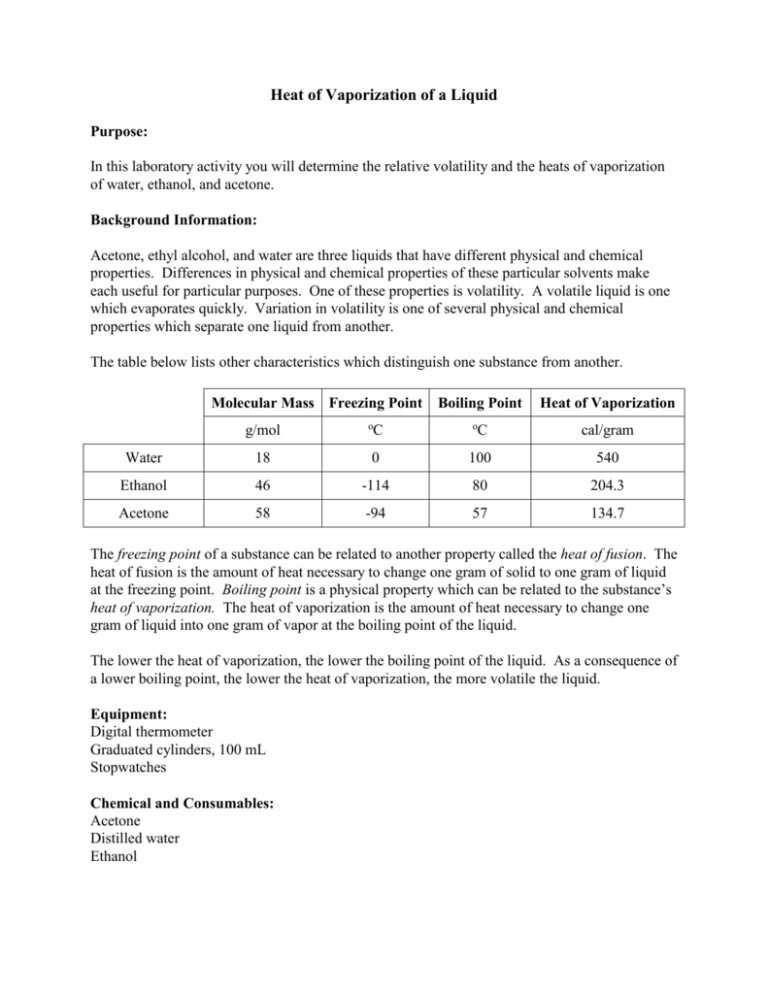
Determining the Enthalpy of Vaporization of Salt Solutions Using the Cooling Effect of a Bubble Column Evaporator | Journal of Chemical Education

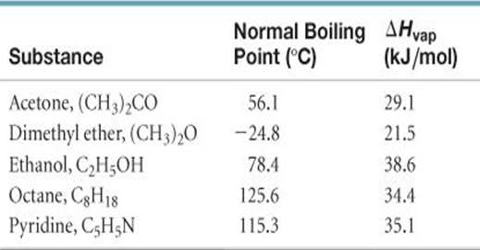

![Solved] Estimate the heat of vaporization (kJ/mol | SolutionInn Solved] Estimate the heat of vaporization (kJ/mol | SolutionInn](https://s3.amazonaws.com/si.question.images/images/question_images/1590/0/8/2/6645ec6bc68ba9271590082649906.jpg)